Phase 2/3 Clinical Trials Begin For Ulotaront To Treat MDD
Major Depressive Disorder (MDD) is predicted to be on top of the global burden of disease by 2030 as depressive disorders affect roughly 280 million people globally. Many patients do not achieve optimal results from their treatment, even with available approaches, but this might change soon for many patients due to this new announcement.
A joint release by Otsuka and Sunovion announced last week that Ulotaront (SEP-363856) has entered a phase 2/3 clinical trial for MDD treatment efficacy. The first patient has been enrolled on 1 December 2022 in a Phase 2/3 clinical study to examine the efficacy of Ulotaront.
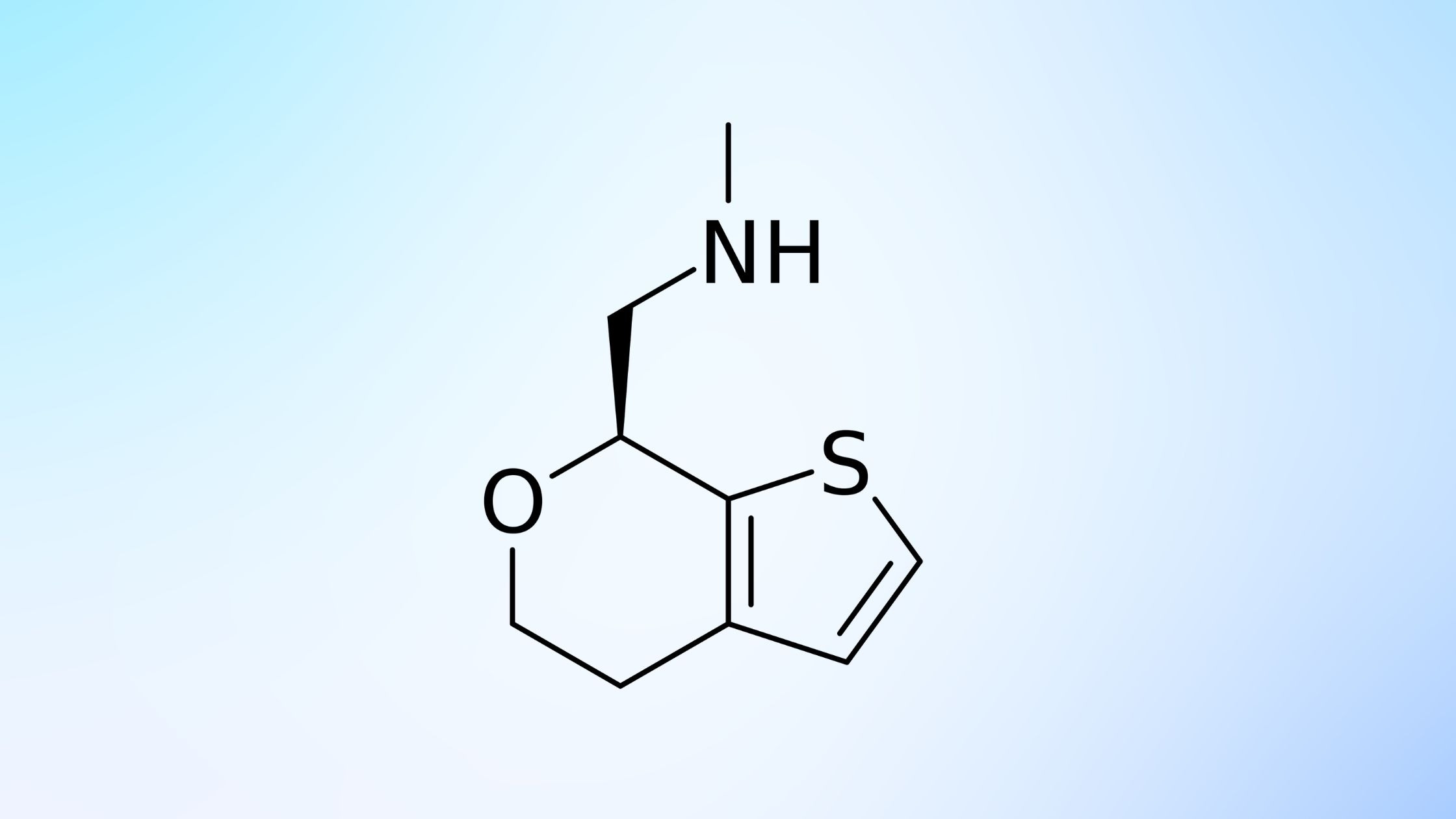
Ulotaront is a trace amine-associated receptor 1 (TAAR1) agonist with 5-HT1A agonist activity to enter phase 2/3 trials as adjunctive therapy for major depressive disorder (MDD), and phase 3 trials for treating schizophrenia in adults and teenagers.
The Ulotaront receptor is the first TAAR1 agonist to be studied as an adjunctive therapy in the treatment of MDD, for which a global, multicenter, randomized, double-blind, placebo-controlled study is developed to evaluate its efficacy, safety, and tolerability.
Patients who do not achieve an optimal response from their current antidepressant treatment will receive Ulotaront or a placebo in addition to their antidepressant therapy, randomly.
What Is The Primary Aim Of The Study?
The primary aim of the study is to reduce depressive symptoms, measured by the change from baseline in MADRS total score, compared to placebo at the end.
Armin Szegedi. M.D., Ph.D., senior vice president, chief medical officer at Sunovion called the initiation of Phase 2/3 to evaluate the use of Ulotaront as an adjunctive treatment of major depressive disorder as a significant step towards exploring the full medical potential of TAAR1 agonist.
He continued that they have been encouraged by the pre-clinical data showing that Ulotaront could be beneficial for those living with mood disorders, and they are looking forward to enhancing the understanding of this ingenious compound in collaboration with Otsuka.

John Kraus, M.D., Ph.D., executive vice president and chief medical officer at Otsuka shared concerns about MDD as it is a disabling mental health condition that often needs first-line antidepressant medication and adjunctive treatments jointly to soothe its symptoms.
In addition, he said that the reason many patients do not achieve optimal responses to treatment is because of the heterogeneous nature of the condition, highlighting the need for new and different treatment modalities.
Otsuka Pharmaceutical Development & Commercialization, Inc. (Otsuka), Sunovion Pharmaceuticals Inc. (Sunovion), and Sumitomo Pharma have jointly developed and commercialized the therapy. Sunovion collaborated with PsychoGenics to discover Ulotaront, a part based on a mechanism-independent approach using the in vivo phenotypic SmartCube® platform and associated artificial intelligence algorithms.
Otsuka was established in 1973 in the United States, and today it has two U.S. affiliates that are Otsuka Pharmaceutical Development & Commercialization, Inc. (OPDC) and Otsuka America Pharmaceutical, Inc. (OAPI) with 2,000 employees. These two companies in the U.S. create and commercialize medicines related to mental health, nephrology, and cardiology, using cutting-edge technology to address unmet healthcare needs.
Sunovion Pharmaceuticals Inc. (Sunovion), based in Mississauga, Ontario, has its headquarters in Marlborough, Mass. It is an indirect, wholly-owned subsidiary of Sumitomo Pharma Co., Ltd.
Sumitomo Pharma Co. Ltd. is the parent company of Ulotaront and is among the top ten listed pharmaceutical companies in Japan. It is operating worldwide in major pharmaceutical markets, including Japan, the USA, China, and other Asian countries with around 7,000 employees globally.
The pharmaceutical firm aims to create innovative pharmaceutical products in the Psychiatry & Neurology area, the Oncology area, and the Regenerative medicine/Cell therapy field. The cell therapy field has been designated as the focus therapeutic area.